What You Need to Know About Multiplex Assays VS. ELISA When Assessing Complex Disease States
ELISA has been the premiere immunoassay technique for decades. But it is problematic for the simultaneous detection of multiple analytes, especially at ultralow concentrations. This kind of detection is now necessary for the accurate and timely clinical assessment of complex diseases or biological processes, involving many biomarkers, from dilute, peripheral biofluids.
Automated assays that simultaneously detect and quantitate multiple analytes at ultralow concentrations are now commercially available. These “multiplex assays” are taking immunoassays to the next level of performance, beyond the limitations of conventional ELISA. Here, you’ll see the current state of the multiplex assay in ELISA.
ELISA is Limited for Modern Diagnostics and Research
While conventional ELISA remains the workhorse for the detection of single analytes in small-scale settings, there is growing demand from research and the clinic for a reliable system for multi-analyte detection and quantification in complex biological processes. Neurological diseases, autoimmune diseases, and cancer require the identification and monitoring of signature cohorts of trace biomarkers. In this context, conventional ELISA can present a number of issues:
- Requires manual processing of complex protocols and data analysis
- Can only detect one analyte at a time.
- Restricts linear dynamic range to only a few orders of magnitude
- Limits colorimetric detection only down to the pg/ml range
Efficient use of time, effort, and physical resources is crucial to modern research. Conventional ELISA’s one analyte detection protocols consume valuable time, effort, reagents, and samples, while limiting throughput and accurate, timely analysis. Today, large cohorts of clinical or drug screening samples demand much more efficient, sensitive, accurate, and versatile immunoassay techniques to assess their inherent biological complexity.

The Multiplex Bead Array Assay Surmounts ELISA’s Limitations
Several varieties of multi-analyte detection array exist today. Planar arrays feature multiple capture and detection antibodies on a two-dimensional format. Flow cytometry-based analysis employs particles bearing reaction sites that can be read via LED or laser light as they file past a detector. Finally, multiplex bead array assays (MBAAs) are immunoassays that use capture beads as their reaction centers. In Simoa® assays , analyte-bound beads get easily parsed to individual wells and sensitively detected via fluorophores, emitting differential wavelengths associated to the beads. Other bead-based technologies maintain a mixed suspension of analyte-bound beads through detection, such is the case with cytometric-based detection methods.
Simoa® MBAAs offers many advantages over traditional ELISA:
- Run more experiments with less labor: Protocols can be largely automated and adapted to high-throughput systems, reducing manual steps, saving researchers time and effort needed for more complex tasks.
- Get more data from less sample: Combines assays for multiple target analytes in a single, smaller reaction volume, reducing workflow, reagent, and sample demands.
- Remain accurate at low concentrations: For each analyte, the capture antibody is conjugated to a bead labeled with a fluorophore emitting at specific wavelength. The immunoassay complex on the multiplex beads is analyzed on the Simoa® disc, allowing detection of each analyte with fg/mL sensitivity. MBAA maintains linearity over nearly 5 orders of magnitude. Whether at fg/ml or ng/ml, your quantitation is spot on.
- Automate data analysis: Automated MBAA systems can gather, process, and display results, using integrated, bespoke, on-board algorithms. Save time and effort, while avoiding manual calculation errors.
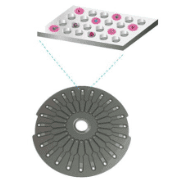
Simoa® MBAAs and Instrumentation Even Surpass Their Multiplex Rivals
In a further contest of multiplex assays vs ELISA, a recent publication1 compared with performance of high sensitivity ELISA against platforms from 3 commercial multiplex assay manufacturers, including Quanterix’s Simoa® magnetic bead-based array assay.
Each system measured specific cytokine markers (IL-1 beta, IL-6, IFN-gamma, and TNF-alpha) in patients with Post-Traumatic Stress Disorder or Parkinson’s disease. Healthy volunteers and clinical populations donated plasma and serum samples. The study characterized the detection limits and precision of each system through several key parameters:
- Sensitivity: measuring the percentage of samples on each platform that had analytes below the limit of quantification (BLQ%), such that lower BLQ% was indicative of better performance.
- Precision: measured by %CVs between duplicate runs in the clinical samples as well as between technical samples plated at different positions within one plate (intra-assay precision) or across the two plates (inter-assay). Across platforms, precision was designated as high when %CV was < 20% or low when %CV was > 75%.
- Overall platform performance: a ranking, based on combined precision (%CV) and relative sensitivity (BLQ%).
Of all the platforms tested, Quanterix’s Simoa® technology had the best sensitivity across all four cytokines for both the plasma and serum samples, exhibited the highest inter- and intra-assay precision, and had the highest sensitivity and precision across all four cytokines.
To quote the authors….
“Overall, the single molecule array (Simoa®) ultra-sensitive platform developed by Quanterix exhibited the best performance across all cytokines and should be recommended for use when highly sensitive and precise immunoassays are required.”
-Heather C.Lasseter, Allison C.Provost, Lauren E.Chaby, Nikolaos P. Daskalakis, Magali Haas, and Andreas Jeromin
Other examples and resources
- Characterizing Links Between Viral Infection And Neurological Manifestations
- Measuring phosphorylated Tau 181, GFAP, NfL and others in blood to transform the way we identify patients with Alzheimer‘s Disease

Ready to take your immunoassays to the next level of performance?
Learn More About Simoa® Technology
References
- Lasseter, H. C. et al. (2020) Cross-platform comparison of highly sensitive immunoassay technologies for cytokine markers: Platform performance in post-traumatic stress disorder and Parkinson’s disease. Cytokine X, 2(2), 100027.