
The Evolution of IFN-a Measurement: How Simoa® is Driving New Breakthroughs
Type I interferons (IFNs) play a central role in the immune response to viral infections. IFN-α, a subclass of the type I IFNs, is comprised of 13 known subtypes that have been the most studied type 1 interferons. Abnormal IFN-α production has been implicated in the pathology of several autoimmune disorders, including systemic lupus erythematosus (SLE), which is characterized by elevated expression of IFN-α in more than half of the SLE patients.1
Despite a critical role, the direct measurement of IFN-α protein in biological samples have been challenging due to minute levels in circulation. Traditional sandwich Enzyme-Linked Immunosorbent Assay (ELISA) methods have proven to lack the sensitivity to reliably quantify IFN-α in blood. In addition, measurement of IFN-α would require multiple ELISA assays for the various subtypes, each with potentially different sensitivity and capacity, making the analysis inefficient and unreliable.
These limitations have led to the development of several assays that measure the IFN-α functional activity, including antiviral activity assays that require the use of virus, reporter gene assays that involve interferon responsive elements, real-time PCR-based assays that measure the expression of a group of interferon stimulated genes (ISGs), and flow cytometry assays that examine surrogate markers.2,3,4,5,6 However, these assays do not provide direct measurement of the IFN-α proteins, limiting deeper insights into IFN-α biology and hindering the translation of IFN-α into a reliable biomarker.
In 2016, an extensive cross-platform study compared nine different technologies and four cytokine immunoassays. The study found that Single Molecule Array (Simoa®) digital immunoassay technology exhibited the highest sensitivity and precision in measuring cytokines in human serum at sub-pg/mL levels. 7 Subsequently, a group of scientists at Institute Pasteur spearheaded the development for a Simoa®-based assay to address the unmet need for measuring ultralow levels of IFN-α. Utilizing Quanterix’s Homebrew Custom Assay Development, this group combined the Simoa® digital immunoassay technology with a selected pair of high affinity antibodies isolated from patients with the autoimmune polyendocrinopathy syndrome type 1/autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APS1/APECED) that harbor antibodies against all IFN-α subtypes. 8 This assay displayed specific reactivity to all IFN-α subtypes and not to other type 1 interferons, with a 5000-fold increase in sensitivity compared to commercial ELISA (Fig. 1), as well as excellent reproducibility and consistency between plasma and serum measurements. Additionally, the IFN-α concentrations measured by the Simoa® assay correlated well with antiviral activity and ISG expression, suggesting that the IFN-α measurement is biologically relevant. 9, 15
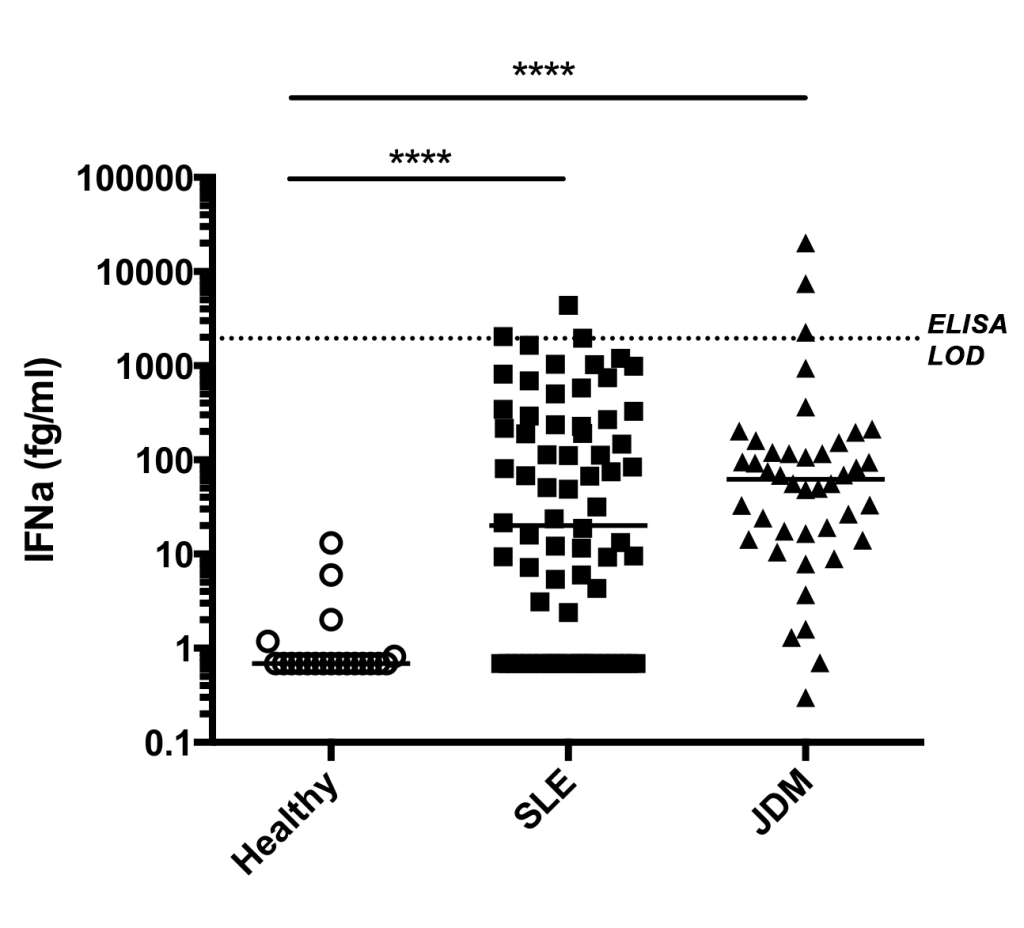
The Simoa®-based ultra-sensitive detection of total IFN-α provided a powerful tool for studying interferon biology at a deeper level. This technology allows for the direct measurement of serum IFN-α, enabling fine mapping of the changes in IFN-α levels across various diseases, disease cohorts, and phenotypical subgroups compared to healthy controls. This has uncovered novel insights into disease-causing pathways. This Simoa®-based assay made it possible to quantify ultralow concentrations of IFN-α in healthy donors and diseased individuals with heterogenous IFN-α expression, revealing an association between higher serum IFN-α and higher degree of clinical severity in SLE patients. Moreover, the ultra-sensitivity of the Simoa®-based assay enabled the identification of disease-specific cellular sources of IFN-α. 9 Using the Simoa®-based IFN-α assay, scientists observed a significant reduction of IFN-α levels in more severe COVID-19 cases, which would be missed with less sensitive immunoassays (Fig. 2).10
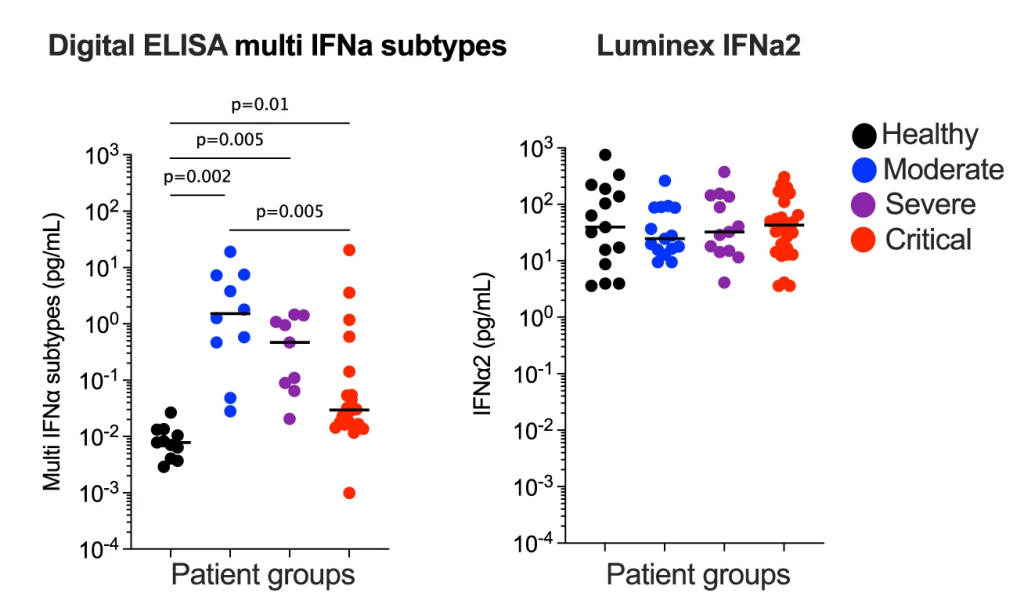
It was also suggested the use of ultra-sensitive technologies, such as Simoa®, may help resolve some of the conflicting results on IFN-α levels reported across many COVID-19 studies. A notable study published in 2023, made possible by the Simoa®-based IFN-α assay, reported that the presence of neutralizing-anti-IFN-Abs in a small fraction of SLE patients was correlated with lower circulating IFN-α levels and better disease outcome. This finding highlighted a role of neutralizing anti-IFN-Abs in modulating SEL pathogenesis and the potential of IFN-targeting therapies for SLE.10
The full potential of IFN-α as a biomarker can only be realized with an assay that is sensitive, reproducible, and scalable. The proxy assays described earlier are either not suited for routine clinical use or warrant further studies.12, 13 Quanterix is now offering a commercial Simoa® IFN-α assay developed with the same antibody pair as in the assays used in multiple ground-breaking studies.9,10,11 The new Simoa® IFN-α Multi-Subtype Advantage PLUS Assay (Quanterix, Cat# 103836), with full automation on the HD-X platform, represents a significant opportunity for the biomedical research community to deepen our understanding of IFN-α biology and accelerate the translation of IFN-α as a biomarker for clinical use.14
Selected References
- Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711-723. doi:10.1084/jem.20021553
- Meager A. Biological assays for interferons. J Immunol Methods. 2002;261(1-2):21-36. doi:10.1016/s0022-1759(01)00570-1
- Lewis JA. A sensitive biological assay for interferons. J Immunol Methods. 1995;185(1):9-17. doi:10.1016/0022-1759(95)00100-o
- Berger Rentsch M, Zimmer G. A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon. PLoS One. 2011;6(10):e25858. doi:10.1371/journal.pone.0025858
- Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906-1916. doi:10.1002/art.21890
- Li Y, Lee PY, Kellner ES, et al. Monocyte surface expression of Fcgamma receptor RI (CD64), a biomarker reflecting type-I interferon levels in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(3):R90. doi:10.1186/ar3017
- Yeung D, Ciotti S, Purushothama S, et al. Evaluation of highly sensitive immunoassay technologies for quantitative measurements of sub-pg/mL levels of cytokines in human serum. J Immunol Methods. 2016;437:53-63. doi:10.1016/j.jim.2016.08.003
- Landegren N, Rosen LB, Freyhult E, et al. Comment on ‘AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies’. Elife. 2019;8:e43578. Published 2019 Jun 27. doi:10.7554/eLife.43578
- Rodero MP, Decalf J, Bondet V, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med. 2017;214(5):1547-1555. doi:10.1084/jem.20161451
- Smith N, Possémé C, Bondet V, et al. Defective activation and regulation of type I interferon immunity is associated with increasing COVID-19 severity. Nat Commun. 2022;13(1):7254. Published 2022 Nov 25. doi:10.1038/s41467-022-34895-1
- Bradford HF, Haljasmägi L, Menon M, et al. Inactive disease in patients with lupus is linked to autoantibodies to type I interferons that normalize blood IFNα and B cell subsets. Cell Rep Med. 2023;4(1):100894. doi:10.1016/j.xcrm.2022.100894
- Enocsson H, Wetterö J, Eloranta ML, et al. Comparison of Surrogate Markers of the Type I Interferon Response and Their Ability to Mirror Disease Activity in Systemic Lupus Erythematosus. Front Immunol. 2021;12:688753. Published 2021 Jun 30. doi:10.3389/fimmu.2021.688753
- Cooles FAH, Isaacs JD. The interferon gene signature as a clinically relevant biomarker in autoimmune rheumatic disease. Lancet Rheumatol. 2022;4(1):e61-e72. doi:10.1016/S2665-9913(21)00254-X
- Mathian A, Mouries-Martin S, Dorgham K, et al. Monitoring Disease Activity in Systemic Lupus Erythematosus With Single-Molecule Array Digital Enzyme-Linked Immunosorbent Assay Quantification of Serum Interferon-α. Arthritis Rheumatol. 2019;71(5):756-765. doi:10.1002/art.40792
- Llibre A, Bondet V, Rodero MP, Hunt D, Crow YJ, Duffy D. Development and Validation of an Ultrasensitive Single Molecule Array Digital Enzyme-linked Immunosorbent Assay for Human Interferon-α. J Vis Exp. 2018;(136):57421. Published 2018 Jun 14. doi:10.3791/57421