
The Brain-Heart Axis: Exploring the Hidden Link Between Cardiovascular and Neurological Health
We often think of the brain and the heart as rivals: one rules our rational decisions, while the other is swayed by emotions. In reality, the two are intricately linked. Researchers have long known that chronic emotional stress can contribute to the development of heart disease and that those who suffer from depression are at higher risk of mortality after acute myocardial infarction (AMI).1 There is even a phenomenon called “broken heart syndrome,” where acute emotional stress, such as a breakup, can cause a temporary weakening of the heart muscle that mirrors symptoms of a heart attack.2
The physiological connection between the brain and the heart is termed the “brain-heart axis” and it describes the bidirectional interactions between these two organ systems, mediated by neuronal, hormonal, immunological and other signaling pathways.3 However, while the comorbidity of cardiovascular disease (CVD) and brain disorders is well recognized, the mechanisms behind it and potential interventions remain elusive (Figure 1). Given the close association between cardiovascular and brain health, neurological plasma biomarkers reflecting brain health could be valuable tools for cardiologists to monitor risks of neurological complications. Assessing neurological biomarkers could help cardiovascular researchers elucidate the mechanisms of the brain-heart connection, predict signs of neurodegeneration, and improve health outcomes for patients.4
Cardiovascular complications of brain injury
Acute brain injury, such as ischemic stroke, hemorrhage, or traumatic brain injury, can cause cardiac dysfunction, including arrhythmia, myocardial damage, and heart failure. Importantly, these cardiovascular complications can occur even in individuals without preexisting heart disease, a phenomenon referred to as cerebral-cardiac syndrome (CCS). CCS is a major cause of post-stroke mortality, driven by mechanisms such as insular cortex injury, autonomic imbalance, catecholamine surges, immune responses, and systemic inflammation.5
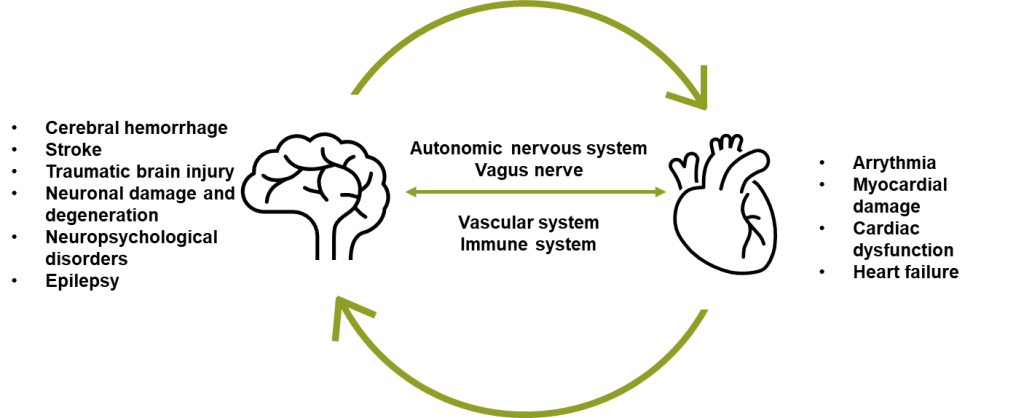
Figure 1: Schematic illustration of the connection between cardiovascular and brain disorders.
Inflammation plays a neuroprotective role during an acute-phase response to neuronal damage; however, prolonged systemic inflammation has a detrimental effect on cellular function. It is hypothesized to be a central player in both CVD and neurodegeneration, influencing the course of disease and rate of progression.6 Neurological, and other blood biomarkers such as cytokines, could be a key to further elucidating the role of inflammation in cardiovascular and neurodegenerative diseases, like Alzheimer’s, as well as the connection between the two.
Alzheimer’s and cardiovascular disease: common companions
Heart health plays an important role in the progression of Alzheimer’s disease (AD) and dementia. Cognitive decline is often viewed as an inevitable symptom of aging. However, there are direct associations between cardiovascular and neurological health. Patients with heart failure often show decreased cognitive function and increased risk of dementia. Researchers postulate that cognitive decline may be linked to impaired cerebral blood flow, systemic inflammation, thromboembolic disease, and protein abnormalities associated with CVD.7
Alzheimer’s disease and cardiovascular disease develop over decades before symptoms appear. Often, heart disease manifests clinically before AD, suggesting it can be viewed as a risk factor or even as a predictor of Alzheimer’s. The two share common risk factors such as smoking, inadequate exercise, APOE4 allele, and high cholesterol and are often preceded by physiological changes such as hypertension and atherosclerosis. Research suggests that up to one-third of AD-related dementia cases may be exacerbated by modifiable atherosclerotic CVD risk factors.6,8
Specifically, cerebral atherosclerosis contributes to poor blood circulation, inflammation, and oxidative stress, which can increase the buildup and reduce clearance of amyloid proteins in the brains of Alzheimer’s patients. Atherosclerosis has been linked to protein expression changes associated with reduced synaptic function, excess myelination, and accumulation of neuronal scaffolding proteins, such as neurofilament light chain (NfL), suggesting neuroaxonal damage.8 In addition, endothelial dysfunction associated with atherosclerosis can disrupt the blood-brain barrier and impede the clearance of amyloid beta (Aβ) and Tau.9, 10
Blood-based biomarkers for better understanding of the brain-heart axis
Ultrasensitive detection methods such as Simoa® (single-molecule array) enable the detection of brain-specific, low-abundance proteins in the bloodstream with high accuracy and specificity.11 Blood-based neurology biomarkers are now widely used in research on neurodegenerative diseases thanks to their accessibility and scalability compared to cerebrospinal fluid (CSF) biomarkers or brain imaging methods.
Biomarkers like NfL and Tau have been used to assess the severity of neuronal injury in heart failure patients and to predict neuronal damage after hypoxic ischemic brain injury following cardiac arrest.7, 12 Other brain injury biomarkers used in brain-heart research include glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl hydrolase L1 (UCH-L1).
Similarly, cardiac biomarkers offer promise for studying the associations between heart and brain health. A meta-analysis of studies published between 2012 and 2022 demonstrated that elevated levels of cardiac biomarkers, including markers of hemodynamic stress and myocardial injury, were linked to structural brain changes and cognitive impairment.13
Quanterix features a full range of individual assays and panels for neurological biomarkers, including total Tau (t-Tau) as well as the novel brain-derived Tau (BD-Tau), NfL, GFAP, among others. The Simoa digital immunoassay technology has delivered the gold standard for ultra-sensitive biomarker detection in serum and plasma, supporting research in neurology, cardiology, and other disease areas. The accessibility of these assays makes them amenable for use in both research and clinical settings, where neurology biomarker testing could become an important part of CVD care for monitoring and predicting neurological conditions.
The future of research in the heart-brain axis
The abundance of research on the heart-brain axis points to the need to explore potential preventive strategies for neurological diseases among CVD patients during the window of opportunity in midlife before these diseases manifest. Blood-based biomarkers play an important role in monitoring the onset and progression of conditions like Alzheimer’s disease and may even lead to the discovery of interventions.
Studying the connection between cardiovascular and brain diseases through the heart-brain axis offers a unique opportunity to uncover key mechanisms driving both conditions through collaboration between cardiovascular and neurology scientists. Insights from this research could pave the way for innovative diagnostics, prevention strategies, and treatments for Alzheimer’s disease, related dementias, and CVD.
Download the Blood Biomarkers for Brain Health brochure to learn more about Quanterix’s neurology biomarkers.
References
- Carney RM, Freedland KE, Steinmeyer B, et al. Depression and five year survival following acute myocardial infarction: a prospective study. J Affect Disord. 2008;109( 1-2 ):133-138. doi:10.1016/j.jad.2007.12.005
- Boyd B, Solh T. Takotsubo cardiomyopathy: Review of broken heart syndrome. JAAPA. 2020;33( 3 ):24-29. doi:10.1097/01.JAA.0000654368.35241.fc
- Simats A, Sager H, Liesz A. Heart brain axis in health and disease: role of innate and adaptive immunity. Cardiovasc Res. Published online August 24, 2024. doi:10.1093/cvr/cvae185
- Hoiland RL, Rikhraj KJK, Thiara S, et al. Neurologic Prognostication After Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-analysis. JAMA Neurol. 2022;79( 4 ):390-398. doi:10.1001/jamaneurol.2021.5598
- Lin HB, Li FX, Zhang JY, et al. Cerebral-Cardiac Syndrome and Diabetes: Cardiac Damage After Ischemic Stroke in Diabetic State. Front Immunol. 2021;12:737170. Published 2021 Aug 27. doi:10.3389/fimmu.2021.737170
- Leszek J, Mikhaylenko EV, Belousov DM, et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr Neuropharmacol. 2021;19( 2 ):152-169. doi:10.2174/1570159X18666200729093724
- Goh FQ, Kong WKF, Wong RCC, et al. Cognitive Impairment in Heart Failure-A Review. Biology (Basel). 2022;11( 2 ):179. Published 2022 Jan 23. doi:10.3390/biology11020179
- Saeed A, Lopez O, Cohen A, Reis SE. Cardiovascular Disease and Alzheimer’s Disease: The Heart-Brain Axis. J Am Heart Assoc. 2023;12( 21 ):e030780. doi:10.1161/JAHA.123.030780
- Duong MT, Nasrallah IM, Wolk DA, Chang CCY, Chang TY. Cholesterol, Atherosclerosis, and APOE in Vascular Contributions to Cognitive Impairment and Dementia (VCID): Potential Mechanisms and Therapy. Front Aging Neurosci. 2021;13:647990. Published 2021 Mar 25. doi:10.3389/fnagi.2021.647990
- Okamoto Y, Yamamoto T, Kalaria RN, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012;123( 3 ):381-394. doi:10.1007/s00401-011-0925-9
- Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28( 6 ):595-599. doi:10.1038/nbt.1641
- Hoiland RL, Rikhraj KJK, Thiara S, et al. Neurologic Prognostication After Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-analysis. JAMA Neurol. 2022;79( 4 ):390-398. doi:10.1001/jamaneurol.2021.5598
- Jensen M, Zeller T, Twerenbold R, Thomalla G. Circulating cardiac biomarkers, structural brain changes, and dementia: Emerging insights and perspectives. Alzheimers Dement. 2023;19( 4 ):1529-1548. doi:10.1002/alz.12926