
Blood Biomarkers for Stroke: Towards Earlier and More Accurate Stroke Diagnosis and Prognosis
In our recent blog post about the brain-heart axis, we explored the link between neurological and cardiovascular health. Along the brain-heart axis, stroke, a major cardiovascular event can have significant implications on acute and longer-term brain health. In this blog post, we delve into the utility of blood-based biomarkers in stroke research, care, and risk management.
Stroke is one of the leading causes of death and disability worldwide. Ischemic stroke (IS) is the most common type of stroke, accounting for about 85% of casualties in stroke patients, while the remainder is primarily attributed to intracerebral bleeding.1 Distinguishing stroke from stroke mimics, having accurate differential diagnosis of stroke subtypes, and timely interventions are essential for improving patient outcomes.
Standard stroke diagnosis relies on neuroimaging techniques such as computed tomography (CT) scan and magnetic resonance imaging (MRI). While these modalities provide critical insights into brain injury, imaging falls short in detecting small lesions and subtle damage associated with acute neuronal damage. MRI requires specialized and expensive equipment, while CT lacks the sensitivity to detect early ischemic changes. Additionally, imaging techniques are often not readily available in emergency settings. These challenges can delay accurate diagnosis and effective treatment, underscoring the need for more rapid and accessible testing methods.2
Blood-based biomarkers offer a promising, more accessible, and cost-efficient alternative that can aid in stroke diagnosis, severity assessment, and longitudinal monitoring. Several categories of blood biomarkers have been studied over decades of research, including lipids, inflammatory markers, hemodynamic markers, microRNAs, metabolites and neurodegeneration markers.3 However, many of them lack the sensitivity and specificity required for clinical use, as evidenced by meta-analysis studies.4 Complex clinical presentation, influences from medications and comorbidities further complicate stroke diagnosis, underscoring the importance of comprehensive, multi-factor characterization of stroke.
Blood-based biomarkers for neuronal damage & neurodegeneration and inflammation can be highly valuable to provide additional insights in differential stroke diagnosis, assessing stroke severity, predicting and monitoring clinical outcomes of brain health. Additionally, blood biomarkers can be utilized in retrospective studies and frequent longitudinal follow-up, for which imaging techniques are not applicable or practical.
Blood biomarkers for stroke-related neuronal damage and neurodegeneration
Stroke patients present a complex and multifaceted pathophysiology, driven by processes such as inflammation and oxidative stress, excitotoxicity, and blood-brain barrier disruption. In addition to causing acute neuronal damage, these processes often lead to stroke-induced secondary neurodegeneration (SNDG), which shares many similarities with neurodegenerative diseases like Alzheimer’s, including neuroinflammation and neuronal loss.5 Specific biomarkers that reflect the brain’s response to the damage could be critical assessing acute brain damage, post-stroke monitoring of brain health, and risk management of SNDG.
Neurological biomarkers, such as glial fibrillary acidic protein (GFAP), Tau, neurofilament light chain (NfL) and S100β, represent important indicators of neuronal damage and neurodegeneration and have shown promise as both diagnostic and prognostic stroke biomarkers.6 A recent study investigated the association of blood-based neurological biomarkers with mortality and adverse event in a large cohort of chronic coronary syndrome (CCS) individuals that had 20-year follow-up data. It was shown that Aβ40/Aβ42 ratio, a biomarker for Alzheimer’s pathology, was related to incident stroke, whereas GFAP was associated with incident stroke in a APOE genotype-dependent fashion.7
GFAP is a biomarker of neuroinflammation, which has been identified as a promising blood-based stroke biomarker candidate in settings where immediate brain imaging is not available.8 Specifically, GFAP levels can help differentiate between hemorrhagic and ischemic stroke. GFAP is rapidly released from damaged glial cells as a result of intracerebral hemorrhage, compared to a slower release in ischemic stroke. This correlation was confirmed by a study demonstrating that GFAP biomarker alone can identify intracranial hemorrhage in patients with suspected stroke.9
NfL is a known marker of neuronal damage and neurodegeneration and has been widely studied in stroke research. Its role as a predictor of functional outcomes after ischemic stroke has been confirmed by various publications. One study showed that plasma NfL levels (but not IL-6 or S100β) could predict clinical severity and subsequent functional outcomes.10 A pooled analysis of five clinical studies confirmed the importance of NfL as a biomarker predicting stroke outcomes and indicated a 1.71 times higher risk of poor functional outcomes in patients with elevated NfL levels.11
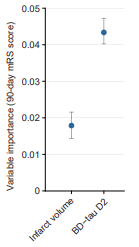
Brain-derived Tau (BD-Tau) is an emerging blood biomarker that’s exhibiting outstanding performance in clinical research for assessing and monitoring brain injury after acute IS. Plasma BD-Tau levels have been shown to correlate strongly with cerebral infarct volumes, a key indicator of brain tissue damage and stroke severity. In a study of 454 and 364 cases from the Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS) and SAHLSIS2, higher acute BD-Tau concentrations were significantly associated with increased odds of unfavorable 3-month functional outcomes based on modified Rankin scale (mRS) score, an established clinical measure of global disability.12 In a related study, plasma BD-Tau concentrations were highly correlated with cerebral infarct volumes and helped improve the prognostic accuracy for poorer ouctomes.13 Using serial blood samples collected at admission, day 2, 3, and 7, researchers presented promising data in a preprint manuscript indicating that plasma BD-Tau reflected acute brain injury at admission and 24-hr infract progression, and that day 2 plasma BD-Tau was superior to imaging-based final infarct volume in predicting the 90-day mRS score (Figure 1).14 These results present very exciting evidence that supports the value of BD-Tau as a highly accurate, accessible and cost-effective biomarker for assessing acute brain injury following ischemic stroke as well as its potential utility for monitoring recovery.
Figure 1. Plasma BD-Tau levels at day 2 showed higher value in predicting 90-day functional outcome compared with final infarct volume determined by delayed neuroimaging. Figure adapted from Voegels, medRxiv 2023.
Inflammatory biomarkers for stroke
In addition to brain-specific biomarkers, inflammatory markers, including C-reactive protein (CRP) and cytokines such as interleukin-6 (IL-6), have been strongly associated with stroke severity and outcome.3,15,16 While neuroinflammation is ubiquitous in post-stroke patients, the treatment of the inflammatory cascade remains largely unaddressed.
A prospective cohort study identified that within 36 minutes after stroke, inflammation markers including plasma IL-6, NfL, ubiquitin C-terminal hydrolase L1 (UCH-L1) and GFAP were elevated by as much as 10 times normal. Inflammatory biomarkers in stroke patients showed a steep increase in the first 2 hours and continued rising for 24 hours.17
Beyond acute intervention, monitoring and mediating the inflammatory response is important during patient recovery. Mouse studies using imaging and histological techniques, along with plasma NfL biomarker, indicate persistent inflammation may contribute to the development of secondary neurodegeneration and therefore presents an important aspect of post-stroke care.18
Capturing the complex pathophysiology of stroke with multi-marker panels
The complex dynamics of biomarker presentation reflect the nuanced pathophysiology of stroke, suggesting a need for comprehensive approaches to enable accurate differential diagnosis, monitoring and appropriate intervention. Multi-marker panels have the potential to capture the different aspects of stroke pathophysiology. Quanterix offers an extensive menu of single and multiplex assay formats across inflammatory (CRP and cytokines) and neurology spectra, such as the Simoa® Neurology 4-Plex D Assay Kit that includes BD-Tau, NfL, GFAP and UCH-L biomarkers as well as the Simoa® Cytokine 4-Plex Assays.
A simple blood test that provides a precise and reliable measurement of biomarkers that aids in stroke diagnosis, predicting severity and prognosis, and guiding treatment decisions will be invaluable for improving stroke care. Monitoring stoke biomarkers can aid not only in diagnosis but in predicting the potential for stroke recurrence, assessing treatment response and identifying patients that would benefit from specific interventions, such as neuroprotective therapies. The Simoa technology enables ultrasensitive, high-throughput biomarker measurement with a fast turnaround time, empowering researchers to accelerate insights into the effect of stroke on the brain in both acute and chronic phases.
To learn how Quanterix’s neurology biomarker assay solutions can power your brain health research download the Blood Biomarkers of Brain Health brochure.

References
- Kuriakose D, Xiao Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int J Mol Sci. 2020;21(20):7609. Published 2020 Oct 15. doi:10.3390/ijms21207609
- Patil S, Rossi R, Jabrah D, Doyle K. Detection, Diagnosis and Treatment of Acute Ischemic Stroke: Current and Future Perspectives. Front Med Technol. 2022;4:748949. Published 2022 Jun 24. doi:10.3389/fmedt.2022.748949
- Anwar L, Ahmad E, Imtiaz M, et al. Biomarkers for Early Detection of Stroke: A Systematic Review. Cureus. 2024 Oct 1;16(10).
- Ruksakulpiwat S, Zhou W, Phianhasin L, et al. A Systematic Review and Meta-Analysis Assessing the Accuracy of Blood Biomarkers for the Diagnosis of Ischemic Stroke in Adult and Elderly Populations. eneuro. 2024 Nov 1;11(11).
- Stuckey SM, Ong LK, Collins-Praino LE, Turner RJ. Neuroinflammation as a key driver of secondary neurodegeneration following stroke? International Journal of Molecular Sciences. 2021 Dec 3;22(23):13101.
- Brunelli S, Giannella E, Bizzaglia M, et al. Secondary neurodegeneration following Stroke: what can blood biomarkers tell us? Frontiers in Neurology. 2023 Sep 1;14:1198216.
- Lohner V, Perna L, Schöttker B, Perneczky R, Brenner H, Mons U. Associations of blood-based biomarkers of neurodegenerative diseases with mortality, cardio- and cerebrovascular events in persons with chronic coronary syndrome. Exp Gerontol. 2025;200:112684. doi:10.1016/j.exger.2025.112684
- Kalra LP, Khatter H, Ramanathan S, et al. Serum GFAP for stroke diagnosis in regions with limited access to brain imaging (BE FAST India). European stroke journal. 2021 Jun;6(2):176-84.
- Jæger HS, Tranberg D, Larsen K, et al. Diagnostic performance of Glial Fibrillary Acidic Protein and Prehospital Stroke Scale for identification of stroke and stroke subtypes in an unselected patient cohort with symptom onset< 4.5 h. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2023 Jan 5;31(1):1.
- Nielsen HH, Soares CB, Høgedal SS, et al. Acute neurofilament light chain plasma levels correlate with stroke severity and clinical outcome in ischemic stroke patients. Frontiers in neurology. 2020 Jun 11;11:448.
- Pekny M, Wilhelmsson U, Stokowska A, Tatlisumak T, Jood K, Pekna M. Neurofilament Light Chain (NfL) in Blood-A Biomarker Predicting Unfavourable Outcome in the Acute Phase and Improvement in the Late Phase after Stroke. Cells. 2021;10(6):1537. Published 2021 Jun 18. doi:10.3390/cells10061537
- Stanne TM, Gonzalez-Ortiz F, Brännmark C, et al. Association of Plasma Brain-Derived Tau With Functional Outcome After Ischemic Stroke. Neurology. 2024 Feb 27;102(4):e209129.
- Gonzalez‐Ortiz F, Holmegaard L, Andersson B, et al. Plasma brain‐derived tau correlates with cerebral infarct volume. Journal of Internal Medicine. 2025 Feb;297(2):173-85.
- Vlegels N, Gonzalez-Ortiz F, Knuth NL, et al. Brain-derived Tau for Monitoring Brain Injury in Acute Ischemic Stroke. Preprint. medRxiv. 2023;2023.11.18.23298728. Published 2023 Nov 19. doi:10.1101/2023.11.18.23298728
- Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005 Jun 1;36(6):1316-29.
- Bustamante A, López-Cancio E, Pich S, et al. Blood biomarkers for the early diagnosis of stroke: the stroke-chip study. Stroke. 2017 Sep;48(9):2419-25.
- Kowalski RG, Ledreux A, Violette JE, et al. Rapid activation of neuroinflammation in stroke: Plasma and extracellular vesicles obtained on a mobile stroke unit. Stroke. 2023 Mar;54(3):e52-7.
- Murphy DP, Dickson DC, Fatema AN, et al. Chronic consequences of ischemic stroke: Profiling brain injury and inflammation in a mouse model with reperfusion. Physiological Reports. 2024 Jun;12(12):e16118.